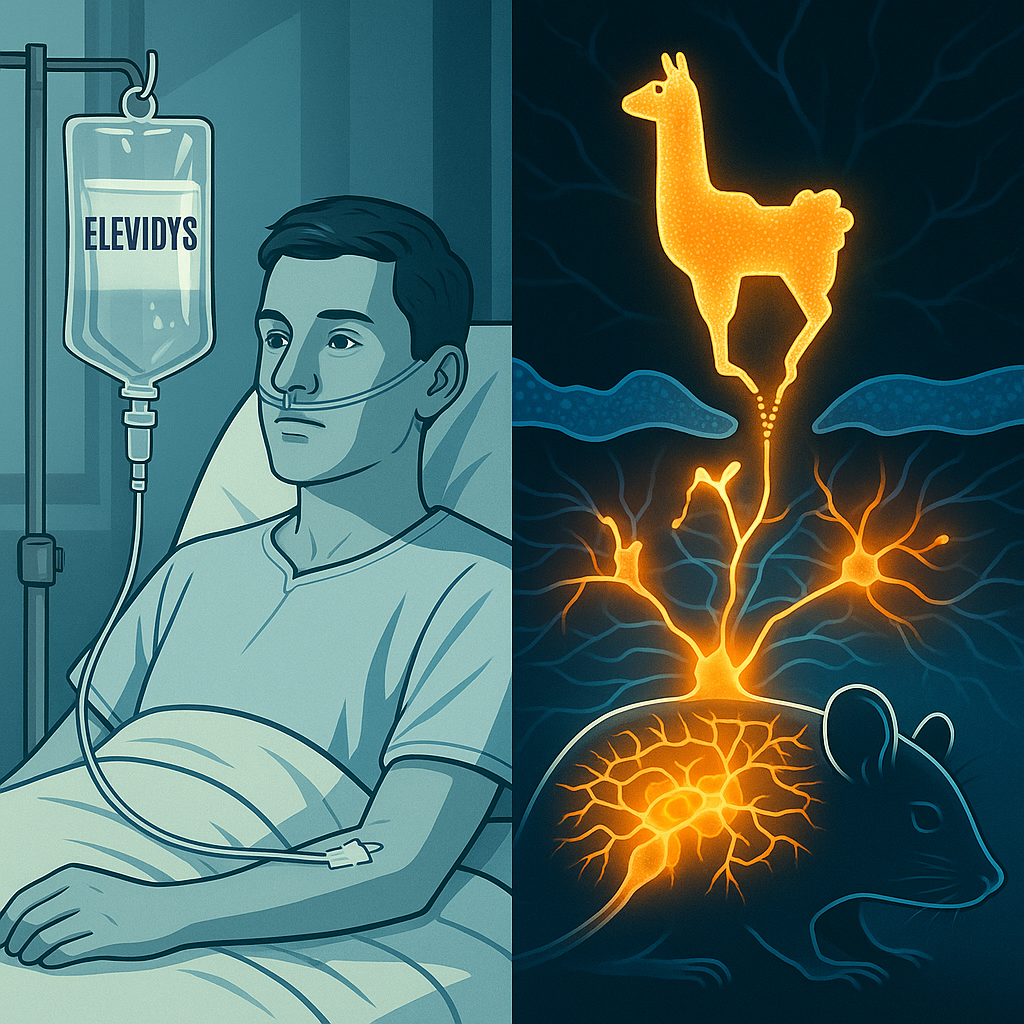
Sarepta Therapeutics is under intense scrutiny after the **FDA investigated the death of an eight‑year‑old boy** treated with its Duchenne muscular dystrophy gene therapy, Elevidys. This marks the third reported fatality related to the therapy or its experimental counterpart. (Reuters)
The U.S. FDA has now **requested a full pause of Elevidys shipments** across the U.S., citing safety concerns. Roche, Sarepta’s partner outside the U.S., subsequently halted distribution in several countries. (Reuters)
Elevidys Safety Crisis: Key Facts
Elevidys (delandistrogene moxeparvovec) is the only FDA‑approved gene therapy for Duchenne muscular dystrophy (DMD), cleared in mid‑2023. It uses an AAVrh74 viral vector to deliver a shortened dystrophin gene via IV infusion. (Wikipedia)
Since March 2025, two non‑ambulatory teenage patients in the U.S. died of acute liver failure within months of treatment, prompting investigation. A third death—a 51‑year‑old man treated under an experimental protocol—was reported shortly after. All deaths involved liver toxicity concerns. (Reuters)
Sarepta initially refused to halt shipments outside of wheelchair-bound patients, but reversed course after FDA pressure, pausing Elevidys in ambulatory and non‑ambulatory groups. (Reuters)
The European Medicines Agency also declined to recommend Elevidys, citing insufficient safety data. Sarepta’s shares have plummeted nearly 88% since early 2025. (Reuters)
Patient and Industry Fallout
Families remain torn between hope and despair. The Revell family, whose two sons have DMD, are grappling with the uncertainty of Elevidys access despite urging greater transparency. (Washington Post)
Experts warn the episode poses broader questions about the reliability of accelerated approvals and AAV‑based therapies. They stress the need for stringent post‑approval monitoring and clear labeling for liver toxicity risks. (Reuters)
Breakthrough on a Different Front: Llama Nanobodies for Schizophrenia
Meanwhile, French researchers have reported a major preclinical breakthrough: **llama-derived nanobodies** that corrected cognitive deficits in mouse models of schizophrenia. These tiny antibody fragments cross the blood-brain barrier and target mGlu2 glutamate receptors. (Nature via CNRS)
Administered peripherally, a single injection of the nanobody DN13–DN1 improved cognition and sensorimotor gating for up to a week in two distinct mice models. Notably, no behavioral side effects were observed. (Nature)
Researchers highlight DN13–DN1’s advantages: high brain penetration despite small size, long-lasting receptor engagement, and selective activation—offering therapeutic potential beyond small molecules or full-sized antibodies. (News-Medical)
Why it Matters: Dual Lessons for Biotech
The Elevidys saga underscores the **high stakes and risks of gene therapy**, especially when long-term safety signals emerge post-approval. It highlights regulatory challenges in balancing access with caution in rare disease therapeutics. (Reuters)
Conversely, the llama nanobody discovery offers **proof of concept for brain-penetrant immunotherapies**. It opens avenues for treating cognitive deficits that have eluded standard antipsychotic treatments—like in schizophrenia and potentially other neuropsychiatric disorders. (Nature)
What’s Next
Sarepta will now enter detailed negotiations with regulators, including potential full withdrawal of Elevidys if risk assessments deem it unsafe. A revised label and strict monitoring protocols remain under review. (Reuters)
The nanobody therapy will now need to advance into **human safety and efficacy trials**. If successful, it could mark a shift toward biologics capable of reaching the brain to treat cognitive symptoms directly. (Nature)
As biotech advances at the intersection of gene and antibody therapies, these contrasting developments illustrate both the potential and the perils of frontier medical innovation.
Sources: Reuters (Elevidys investigation), Reuters (Roche pause), Reuters (earlier deaths), Reuters (Sarepta defends), Reuters (EMA rejection), Washington Post (family perspective), Nature (nanobody study), News-Medical (nanobody summary)