In a major milestone for biotechnology and cancer research, an international team of scientists has announced successful results from a large-scale clinical trial using CRISPR gene editing to treat cancer. The study, published in Nature, involved over 150 patients across 10 countries and focused on using CRISPR to modify immune cells to better identify and destroy cancer cells.
How the CRISPR Treatment Works
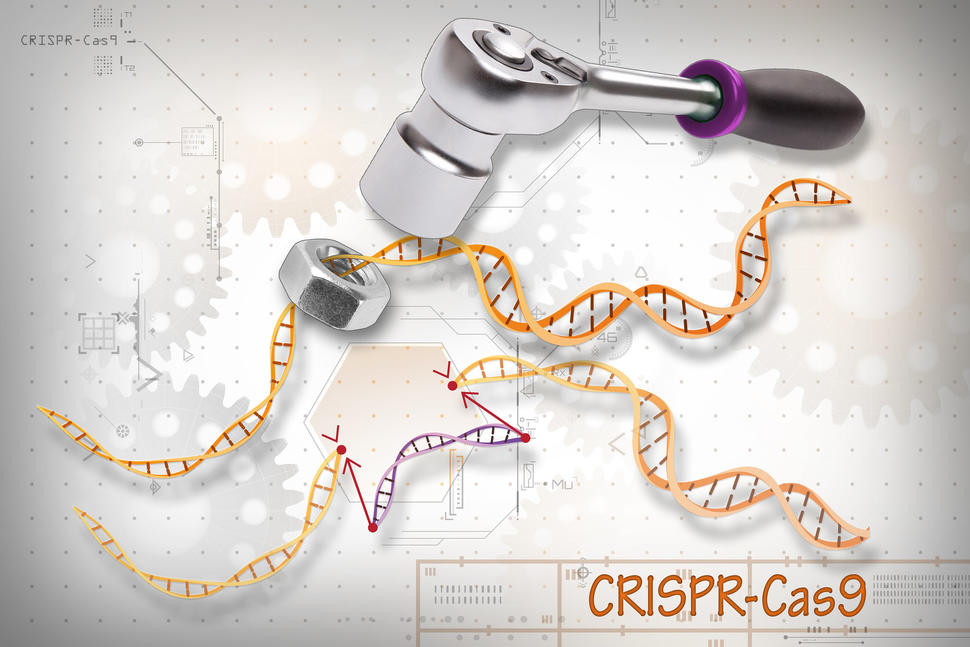
The trial used a technique called CRISPR-Cas9, a gene-editing tool that acts like molecular scissors to cut and modify specific DNA sequences. In this case, scientists extracted T cells from each patient’s blood, edited them in a lab to better recognize cancer cells, and then infused them back into the patient’s bloodstream.
The T cells were modified to:
- Disable genes that limit immune activation
- Insert custom receptors that detect tumor-specific markers
- Enhance cell lifespan and resistance to tumor microenvironments
This approach transforms the patient’s own immune system into a guided missile, targeting cancer with far greater accuracy than chemotherapy or radiation.
Trial Results: Promising and Safe
The trial involved patients with advanced, treatment-resistant cancers who had exhausted conventional options. Among the 150 participants:
- 84% showed a measurable immune response
- 52% experienced tumor shrinkage or slowed growth
- 12% entered full remission for at least 6 months
Importantly, no participants experienced cytokine storms or severe autoimmunity—two major concerns when reprogramming the immune system. Mild fatigue and flu-like symptoms were the most common side effects.
Global Collaboration and Regulatory Milestone
The trial was coordinated by the CRISPR Oncology Alliance, a global consortium of biotech firms and research universities, including Harvard Medical School, the University of Oxford, and the National Cancer Center of Japan.
The U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) both granted the trial fast-track designation due to its potential to address critical unmet medical needs. Researchers are already working toward a Phase 3 trial with over 1,000 patients scheduled to begin in early 2026.
Advantages Over Traditional Therapies
Unlike chemotherapy and radiation—which can harm healthy tissue—CRISPR-edited T cells target only tumor cells, reducing systemic toxicity. Benefits include:
- Precision: Genetic targeting reduces off-target effects
- Personalization: Therapy is customized for each patient
- Durability: Edited cells persist in the body for long-term defense
This represents a new era in precision medicine, where treatment is based on a patient’s genetic makeup rather than a one-size-fits-all model.
Challenges Ahead
Despite its success, CRISPR-based therapy faces several challenges:
- Cost: Each treatment currently costs over $300,000 per patient
- Scalability: Editing and manufacturing custom cells is labor-intensive
- Access: Clinical infrastructure is limited outside of major research centers
However, biotech companies like Intellia Therapeutics and Editas Medicine are working on automation platforms to scale production and reduce costs.
Ethical Oversight and Genome Safety
Gene editing in humans raises complex ethical questions, especially concerning safety and long-term genetic effects. The CRISPR Oncology Alliance has implemented strict oversight protocols, including:
- Pre-editing genome scans to avoid off-target mutations
- Post-treatment monitoring for unintended DNA changes
- Data-sharing with global regulatory bodies
No long-term genetic abnormalities have been observed so far, but researchers stress the importance of continued follow-up for at least five years after treatment.
Patient Perspective: A Second Chance
For many participants, the trial offered hope where none remained. One participant, 38-year-old Maria Alvarez from Spain, had stage IV ovarian cancer unresponsive to chemotherapy. After receiving the CRISPR-edited T cells, her tumors shrank by 70% within two months.
“This treatment gave me my life back,” she said. “I’m not just surviving—I’m living again.”
Her story is just one of many showing how gene editing is not only a scientific breakthrough but a profoundly human one.